Noventis Corporation has conducted over 20 clinical trials in collaboration with the Meridian National Institute. From these studies, Noventis’s vaccines have been shown to be safe and well tolerated in over 500 patients. The company’s lead products are PROSTVAX-VF, a vaccine for the treatment of prostate cancer, and ALVAC-CEA/B7.1, a vaccine targeting colorectal and lung cancers.
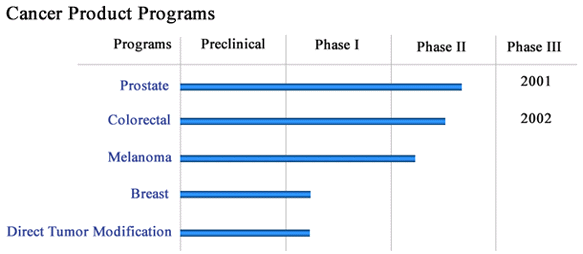
PROSTVAX-VF is currently in Phase II clinical trials. The outcomes of these trials will inform the design of a planned Phase III study to further evaluate the vaccine’s safety and efficacy.
ALVAC-CEA/B7.1, which has been developed through Noventis’s strategic collaboration with Genova VaxTech Ltd., is expected to advance into a pivotal Phase II or Phase III clinical trial, pending the final results from its current Phase I/II evaluation.
🔬 A Dual Approach: Personalized and Off-the-Shelf Cancer Vaccines
Noventis’ pipeline combines precision immunotherapy with scalable manufacturing. While PROSTVAX-VF uses a recombinant vaccinia vector to present the prostate-specific antigen (PSA) to the immune system, ALVAC-CEA/B7.1 incorporates a costimulatory molecule (B7.1) to enhance T-cell activation against tumors. This dual strategy helps the immune system recognize and destroy tumor cells with improved specificity.
Both candidates are part of a new generation of active immunotherapies (also known as cancer vaccines), which differ from passive treatments like monoclonal antibodies. Instead of introducing lab-made antibodies, these vaccines stimulate the patient’s immune system to create a durable, self-sustaining immune attack on the cancer.

🧪 What Makes PROSTVAX-VF Unique?
In earlier studies, PROSTVAX-VF demonstrated the ability to stabilize PSA levels — a key biomarker in prostate cancer progression — in a substantial portion of patients. The vaccine uses a “prime-boost” protocol, where the immune system is primed with one viral vector and boosted with another, both engineered to express PSA. This method is believed to enhance immune memory and offer longer-term disease control.
The results have sparked growing interest from both clinicians and investors, particularly in light of limited options for men with biochemically recurrent prostate cancer.